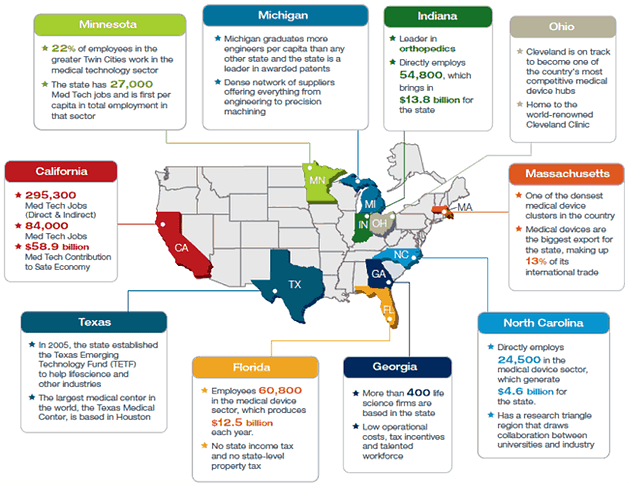
A Business Plan Number Does Not a Marketing Plan Make
Companies often spend weeks if not months working out their business plans for the coming year. The presentations get written, presented to ‘The Board”, get reviewed, critiqued and then re-written. The focus of the critique and the